What is a Semiconductor?
A semiconductor is a material which falls between a conductor and insulator. Its resistance decreases with increase in temperature. The naturally existing Semiconductors has four Electrons. Some of the semiconductor materials are Silicon, Germanium and Gallium Arsenide. The most commonly used material is Silicon.
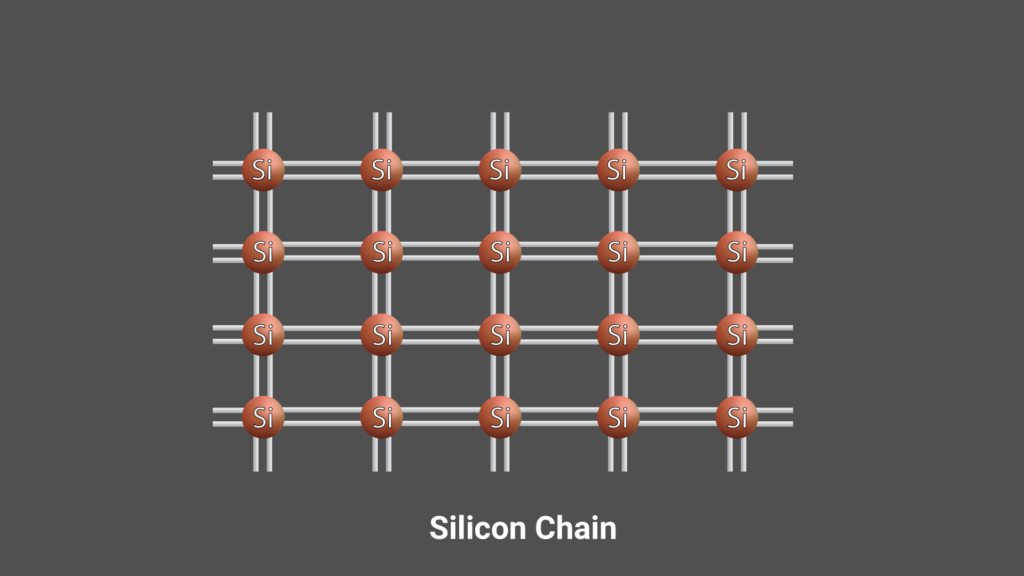
The semiconductors are classified into N-Type and P-Type Semiconductors based on Dopants added.
N-Type Semiconductors:
These type of Semiconductors are made by doping pentavalent atoms in a four electrons crystal structure. The pentavalent dopants used in semiconductors are Antimony, Phosphorus, and Arsenic. In this type of semiconductor, the extra one electron from the dopant plays a major role in conductivity.
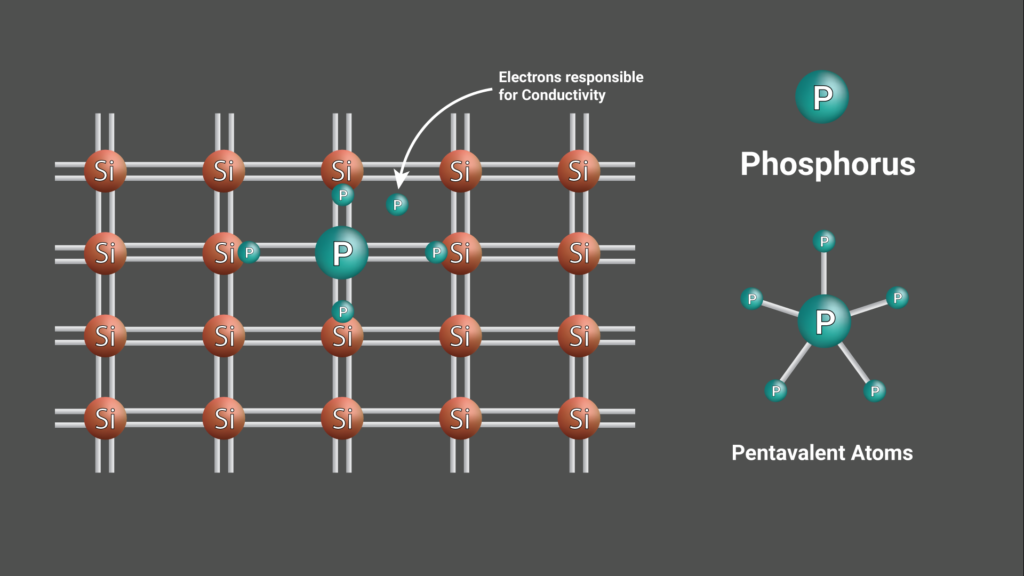
P-Type Semiconductors:
These type of Semiconductors are made by doping trivalent atoms in a four electrons crystal structure. The trivalent dopants used are Boron, Gallium, and Indium. In this type of semiconductor, the vacant one region of an electron is called holes, which plays a major role in conductivity.
These type of doped Semiconductors are called Extrinsic Semiconductors.
Applications:
- It is widely used in diodes, transistor and other modern-day electronics (Processors, Chips)
- It is even used in photovoltaic cells (Solar Panels)
- It is used to make Light Emitting Diodes.
- It is also used in Quantum dots (tiny semiconductor particles a few nanometres in size, having optical and electronic properties)